
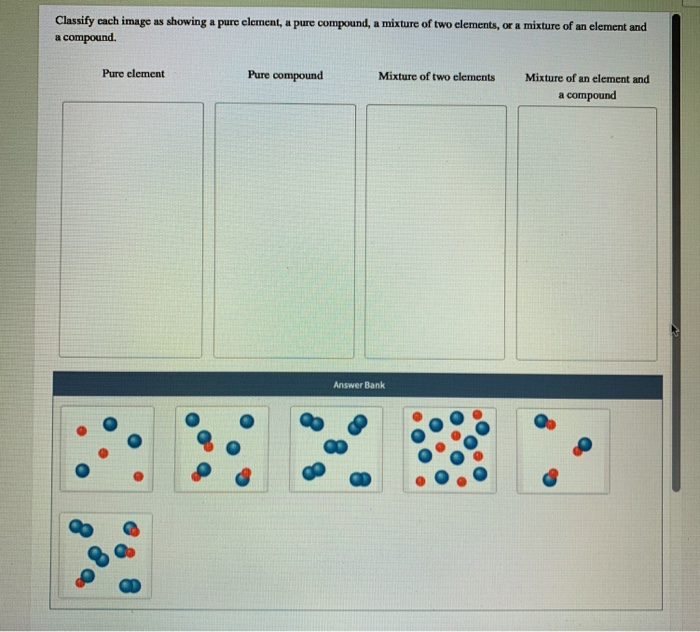
For the latter choice, identify as homogeneous or heterogeneous As always, if your teacher differs from my answer, go with your teacher's answer.Įxample #3: Identify each example as a compound, element, or mixture. This is because ALL samples of elements or compounds contain impurities. I might also add that some teachers would consider diamond to be pure carbon, therefore identifying it as a pure substance. Here is some more discussion on this topic. During the formation process, impurities are included in random areas of the diamond crystal. You might think that diamond is a pure substance, but keep in mind that it is a substance formed in nature. (Consider naturally-formed diamond, however, to be a heterogeneous mixture.) (f) carbon -> all chemical compounds are pure substances. (e) liquid nitrogen -> N 2 (liquid, gas, or solid) is a chemical element. (d) marble stone -> this is a mixture, a heterogeneous mixture to be specific. (c) diamond -> solid carbon, but there are impurities. (b) table sugar -> 100% sucrose, a chemical compound. Solution: (a) dry ice -> solid CO 2, a chemical compound. (a) dry ice (b) table sugar (c) diamond (d) marble stone (e) liquid nitrogen (f) carbon They are different, which is the hallmark of a heterogeneous mixture.Įxample #2: Which of the following would best be described as a mixture? Is it heterogeneous or homogeneous? Break off two different chunks, then compare them. Not all areas in the cookie are the same. Remember, all chemical elements and chemical compounds (when not mixed with anything else) are considered to be pure substances.īaking soda is 100% sodium bicarbonate, a chemical compound. However, that is fairly rare in these moden times of ours. Sometimes, tap water that has been improperly treated will have little bits of solid stuff. This protects underground water pipes (those made of iron) from corrosion.

Also, tap water is treated so as to produce a pH of about 10. It may have some residual chlorine from the water treatment plant. I advise you to not question them on this point. It may be that your teacher insists that table sugar is a pure substance because they believe it to be 100% sucrose (which is a legitimate chemical compound). I think it might be mostly glucose and/or fructose. I have not been able to find information on the composition of table sugar, so I can't really comment on what the other 0.3% is. Table sugar is about 99.7% sucrose (a chemical compound). Here is an example of the contents of this mixture.
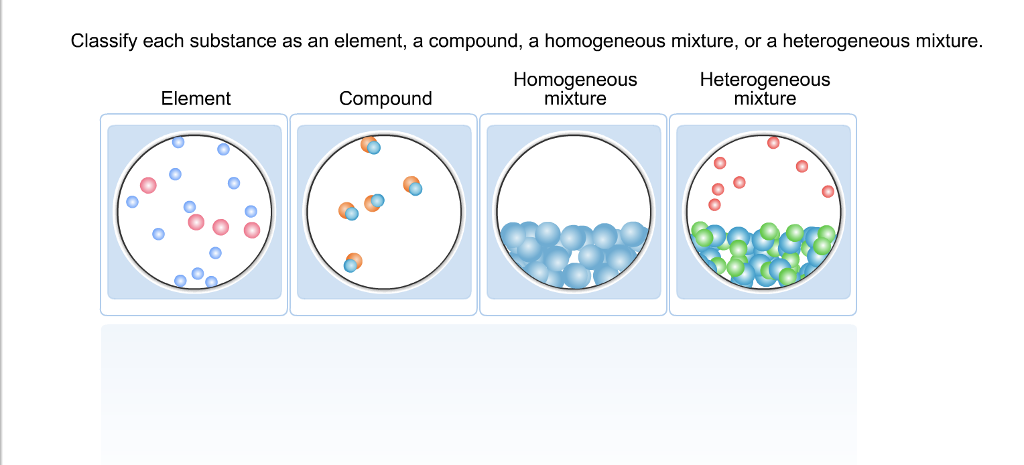
The powdered drink is mostly sugar, with a number of other chemicals for taste and to retard spoilage. Pure substance, specifically an element (sometimes the term 'chemical element' is used). You should NOT use this to claim that there are no true pure substances. Pure substance, specifically a chemical compound.īy the way, ALL chemical elements and compounds possess very small amounts of impurities. This is ignored when classifying aluminum as a pure substance. If it happens to you, go with what the teacher wants.īy the way, a sample of aluminum, no matter how pure, will always have a tiny bit of non-aluminum impurity. That individual is operating under the misapprehension that table salt is 100% NaCl. Here is a brief discussion about chemicals added to table salt.īe careful: in very rare instances, a teacher may require you to answer that table salt is a pure compound. Table salt is 97 to 99% sodium chloride with a variety of other chemicals added in. (f) table sugar (g) tap water (h) gold (i) baking soda (j) chocolate chip cookie (a) table salt (b) aluminum (c) NaCl (d) magnesium (e) powdered orange drink Pure substance or mixture - 20 single-part examplesĮxample #1: Classify each example as either a pure substance (an element or a compound) or mixture (heterogeneous or homogeneous). ChemTeam: Pure substance or mixture? Pure substance or mixture?


 0 kommentar(er)
0 kommentar(er)
